Last updated on August 11th, 2025 at 12:25 am
Dilution, a fundamental concept in various scientific fields such as chemistry, microbiology, and biochemistry, involves the process of reducing the concentration of a solution by adding more solvent. This process is crucial for achieving accurate experimental results and preparing solutions of desired concentrations. This article will explore How to calculate Dilution Factor across different applications.
The Dilution Factor is not a small topic. This is a huge topic in itself, and to cover this topic, we will have to answer thousands of questions related to it. We have given such information earlier in our blog, New York Universities. In the previous article, we told you which universities are best for information technology in New York. Similarly, in this article, we will tell you how to calculate the Dilution Factor of different types of substances. So, friends, let’s start the article.
What is the Dilution Factor in Chemistry?
In chemistry, the dilution factor is the ratio of the final volume of a solution to the initial volume of the concentrated sample before it was diluted. It tells you how much the original sample has been diluted.
(( This information was obtained with the assistance of the website Differencebetween.com. For further details, you can visit the website. ))
What is the Dilution Factor in Microbiology?
In microbiology, the dilution factor is used to describe how much a sample (like bacteria or other microorganisms) has been diluted by adding a liquid. It helps in calculating the original concentration of microorganisms in a sample after dilution.
To determine the dilution factor for each tube in the dilution series, one must multiply the individual dilution factor for the specific tube by the product of all previous tubes in the series. This comprehensive calculation ensures an accurate representation of the dilution levels throughout the series, providing essential information for various microbiological analyses.
(( This information was obtained with the assistance of the website American Society for Microbiology. For further details, you can visit the website. ))
What is the Dilution Factor in Biochemistry?
Dilution in biochemistry refers to the process of reducing the concentration of a solute in a solution, typically achieved by adding more solvent, such as water, to the solution. Essentially, diluting a solution involves increasing the solvent quantity without introducing additional solute.
The concept of dilution factor in biochemistry aligns closely with its counterpart in chemistry, as discussed earlier.
Calculating Dilution Factor in Biochemistry:
The Dilution Factor can be calculated using the following formula:
Dilution Factor (DF) = Vfinal / Vinitial
Where:
- Vfinal is the final volume of the diluted solution.
- V initial is the initial volume of the concentrated solution.
For instance, if you mix 1 mL of a concentrated solution with 9 mL of a solvent to create a total volume of 10 mL, the Dilution Factor would be 10 (10 mL total volume / 1 mL initial volume).
(( This information was obtained from Wikipedia. For further details, you can visit the website. ))
What is the Formula for Dilution Factor Concentration?
The formula to calculate dilution factor concentration is simple yet powerful: Dilution Factor (DF) = Final Volume (Vf) / Initial Volume (Vi). This formula applies universally to all dilution scenarios, from chemistry experiments to microbiological assays. The dilution factor quantifies the degree of dilution applied to the original solution.
Is the Dilution Factor Positive or Negative?
The dilution factor is always positive because it represents a ratio of volumes (final volume divided by initial volume). Since volumes are positive values, the dilution factor itself will always be a positive number.
Is the Dilution Factor a Whole Number?
Yes, the dilution factor is typically expressed as a whole number or a simple fraction. This is because dilution is achieved by combining whole units of the original solution with whole units of the solvent. Fractions or decimal values are impractical for dilution factors, as they may result in non-standardized volumes.
Are Dilution Factors Additive?
Dilution factors are not additive. When performing multiple dilutions in sequence, the individual dilution factors are multiplied together to determine the overall dilution factor.
For example, if you perform a 1:10 dilution followed by a 1:100 dilution, the overall dilution factor would be 1:1000.
How to Calculate Dilution Factor?
Calculating a dilution factor is straightforward. Simply divide the final volume (after dilution) by the initial volume (before dilution). For instance, if you start with 5 mL of a solution and add 45 mL of solvent, the dilution factor is 50 (50 mL final volume / 1 mL initial volume).
How to Calculate Dilution Factor of Serial Dilution?

Calculating the dilution factor of a serial dilution involves determining the overall dilution resulting from a series of consecutive dilutions. Serial dilutions are often used in laboratories to achieve a desired concentration range from a stock solution. To calculate the dilution factor of a serial dilution, follow these steps:
- Understand the Concept: In a serial dilution, multiple dilutions are performed in a sequence. Each dilution step contributes to the overall dilution factor.
- List the Dilution Steps: Write down the dilution steps involved in the serial dilution. For example, you might perform three consecutive 1:10 dilutions.
- Calculate Dilution Factors for Each Step: Calculate the dilution factor for each step by using the given ratio of the dilution. For a 1:10 dilution, the dilution factor is 10.
- Multiply Dilution Factors: Multiply the dilution factors of all the consecutive steps to find the overall dilution factor. For example, if you have three 1:10 dilutions, the overall dilution factor would be 10 × 10 × 10 = 1000.
- Interpreting the Result: The calculated overall dilution factor represents how much the original solution has been diluted throughout the entire series of dilution steps.
Here’s an example to illustrate:
Suppose you have a serial dilution of 1:10, 1:100, and 1:1000. To calculate the overall dilution factor:
| Dilution Step | Dilution Factor | Cumulative Dilution Factor |
|---|---|---|
| Step 1 | 10 | 10 |
| Step 2 | 10 | 10 × 10 = 100 |
| Step 3 | 10 | 100 × 10 = 1000 |
How to Calculate Dilution Factor in HPLC?
In High-Performance Liquid Chromatography, the dilution factor is calculated by dividing the initial concentration of the analyte by the final concentration after dilution. This factor is crucial for accurately quantifying the concentration of a compound in a sample.
How to Calculate Dilution Factor in Titration?
In titration experiments, the dilution factor is determined by the volume of the analyte solution and the volume of the titrant added. Dividing the final volume by the initial volume yields the dilution factor. This factor aids in determining the molarity of the analyte.
How to Calculate Dilution Factor of the Viable Plate?
In microbiology, the Dilution factor for viable plate counting is calculated by dividing the volume of the sample added to the agar plate by the total volume of the diluted sample. This factor helps determine the concentration of viable microorganisms in the original sample.
How to Calculate Dilution Factor in a Spectrophotometer?
When using a spectrophotometer to measure absorbance, the dilution factor is determined by the dilution of the sample and the path length of the cuvette. Dividing the initial concentration by the final concentration gives the dilution factor.
How to Calculate Dilution Factor of Water?
Calculating the dilution factor for water involves dividing the final volume of the diluted water solution by the initial volume of the concentrated solution. This factor is essential for preparing solutions of desired concentrations while maintaining accurate proportions.
How to Calculate Dilution Factor in RBC Count?
In RBC count, the dilution factor is calculated by dividing the volume of the diluted blood sample by the volume of the original blood sample. This factor is vital for accurately determining the concentration of RBCs in the blood.
For instance, if you dilute 0.1 mL of blood with 9.9 mL of diluent to get a total volume of 10 mL, the dilution factor would be 100 (10 mL total volume / 0.1 mL initial volume).
How to Calculate Dilution Factor in WBC Count?
In the WBC count, the dilution factor is calculated similarly to the RBC count. Divide the volume of the diluted blood sample by the volume of the original blood sample to obtain the dilution factor. This factor is essential for accurately estimating the concentration of WBCs in the blood.
How to Calculate Dilution Factor for Cell Counting?
For cell counting experiments, the dilution factor is determined by dividing the volume of the diluted cell suspension by the volume of the original cell suspension. This factor helps ensure that the cells are properly dispersed and counted, yielding accurate results in cell concentration determination.
How to Calculate Dilution Factor for DNA Concentration?
In DNA concentration determination, the dilution factor is calculated by dividing the volume of the diluted DNA solution by the volume of the original DNA solution. This factor is crucial for accurately quantifying the concentration of DNA, which is vital in various molecular biology experiments.
How to find the correct dilution factor stats?
To find and apply the correction for the dilution factor in statistics or scientific experiments, you need to follow a systematic process. Here’s how you can calculate and correct the dilution factor:
Calculate the Dilution Factor:
The dilution factor is the ratio of the final total volume of the diluted solution to the initial volume of the undiluted sample. The formula for the dilution factor is:
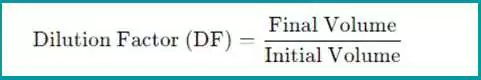
- Final Volume: The total volume after dilution (sample + solvent).
- Initial Volume: The volume of the original sample before dilution.
Example:
- If you dilute 1 mL of a sample into 9 mL of solvent, the final volume becomes 10 mL.
- The dilution factor would be:

Measure the Concentration or Value in the Diluted Sample:
After performing the dilution, you’ll usually measure a concentration or some value from the diluted sample. This value will be lower due to the dilution, and you’ll need to adjust it to reflect the original concentration in the undiluted sample.
Example:
- Let’s say you measure a concentration of 2 mg/L in the diluted sample.
Correct for the Dilution Factor:
To correct the measured value and find the actual concentration in the original undiluted sample, multiply the measured value by the dilution factor. The formula for the corrected concentration is:
Corrected Concentration=Measured Concentration×Dilution Factor
Example:
- If the measured concentration in the diluted sample is 2 mg/L and the dilution factor is 10, then the actual concentration in the undiluted sample is:
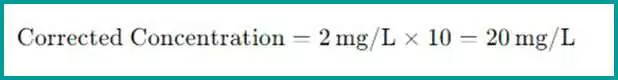
Conclusion.
The concept of dilution factor plays a pivotal role in numerous scientific disciplines, including chemistry, microbiology, and biochemistry. Understanding how to calculate dilution factors is essential for preparing solutions of desired concentrations, estimating cell counts, and quantifying analytes accurately.
Whether it’s a simple 1:10 dilution or a complex serial dilution, the principles remain the same – divide the final volume by the initial volume to determine the dilution factor.
Faq’s
What is dilution factor 1 to 5?
1:5 dilution factor involves combining 1 part of a solution with 5 parts of a diluent.
What is a 1 to 100 dilution factor?
1:100 dilution factor entails mixing 1 part of a solution with 100 parts of a diluent.
How do you calculate a 1 to 20 dilution factor?
To calculate a 1:20 dilution factor, divide the final volume (20) by the initial volume (1), resulting in a dilution factor of 20.
Thanks for your visit.
(How to Calculate Dilution Factor?)
Disclaimer: The information in this article is for general knowledge. Before taking any action, consult a qualified expert. The author and the website disclaim liability for decisions based on this article. Seek professional advice for specific guidance.


How do i calculate Dilution factor by myself. Please tell me